New paper: Modelling a bistable switch underlying commitment to apoptosis
The Bcl-2 family of 15 or more proteins are key regulators of the intrinsic apoptosis pathway. Determining the mechanism two of these proteins (Bak and Bax) use to control mitochondrial outer membrane permeabilisation (MOMP) and subsequent cytochrome c release is therefore the focus of significant research.
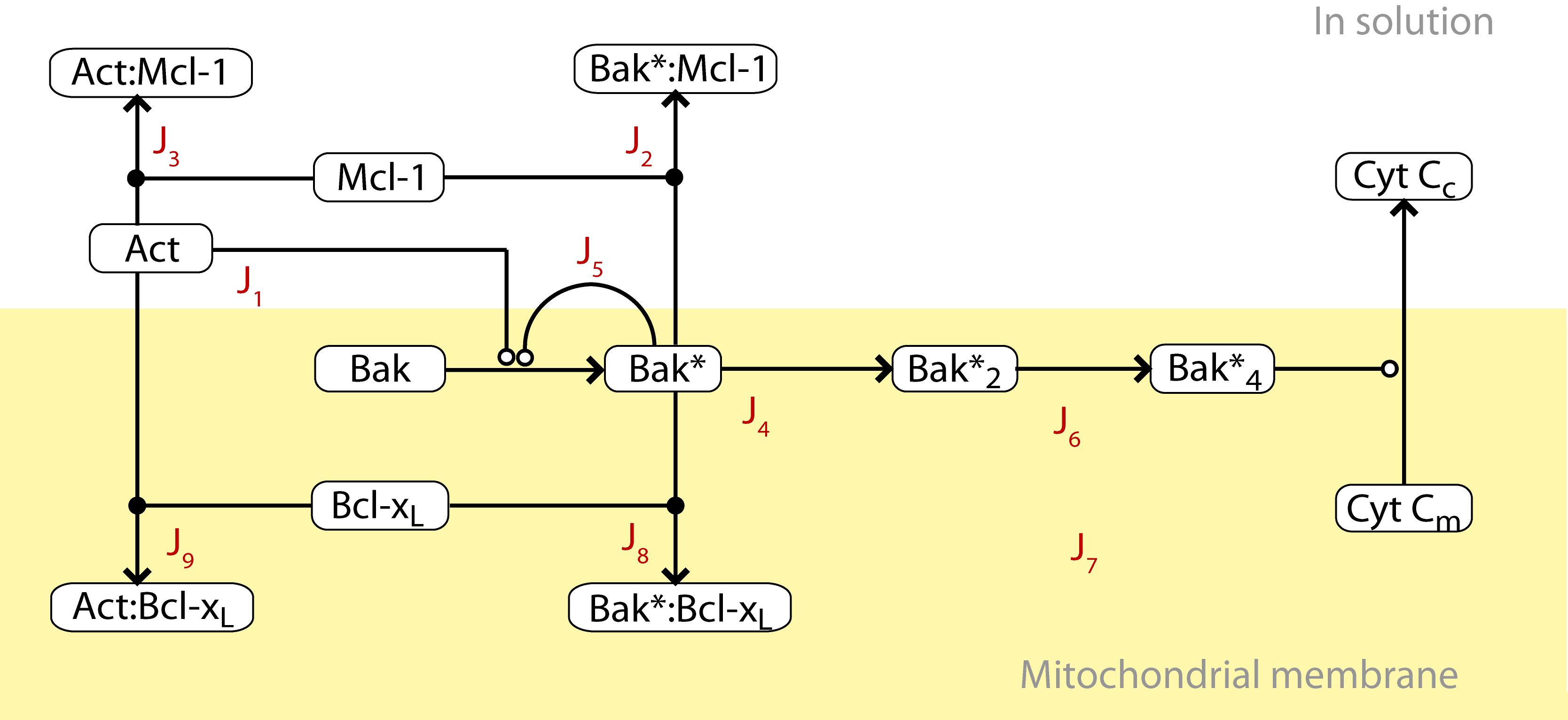
In collaboration with Ruth Kluck, Terry Speed, and others at WEHI, I made a deterministic mass-action model of a subset of Bcl-2 family protein interactions is constructed in order to better understand a reduced mitochondrial system in vitro, and its role in apoptosis in vivo. A model which includes direct activation of pro-apoptotic Bak by BH3-only effector proteins is shown to be more consistent with kinetic binding data and Mice Liver Mitochondria (MLM) experiments, compared with a model which does not include direct activation.
This represents a novel in vitro model of Bcl-2 mediated apoptosis constrained by experimental and kinetic data. The model does not regulate MOMP through the existence of a bistable switch, as posited by other computational studies. The robustness of the model to parameter variation highlights the different roles pro-survival proteins may play depending on the BH3-only stimulus.
Lansdell B, Kluck R, Hockings C, Lee E, Fairlie D, Frascoli F, Landman K, Speed T, BMES 2013